History
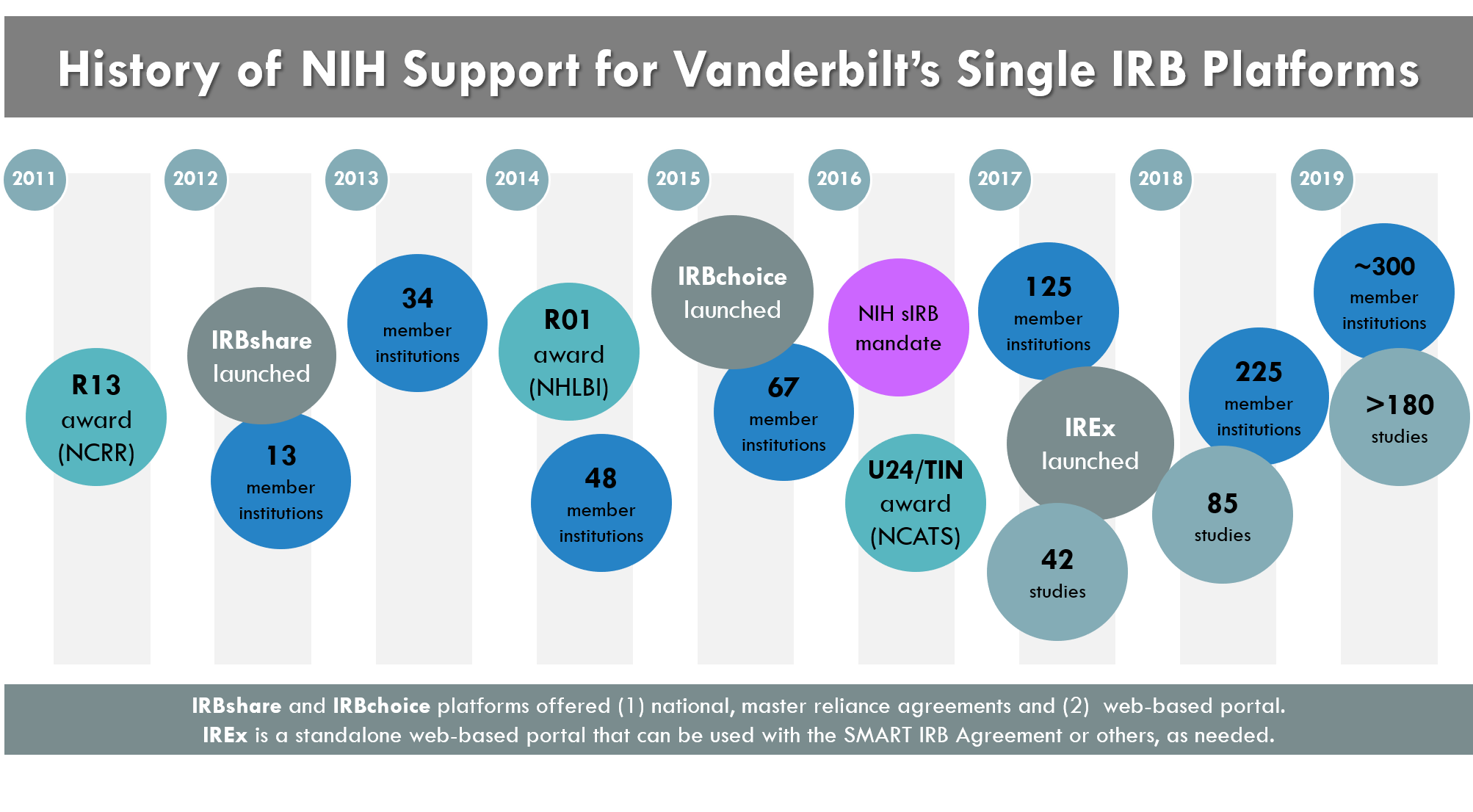
The IRB Reliance Exchange (IREx) portal was launch in February 2017 by Vanderbilt University Medical Center as part of the Duke-Vanderbilt Trial Innovation Center and the larger Trial Innovation Network.
IREx Evolved from Other NIH-supported Initiatives
- In 2011, Vanderbilt was awarded an R13 conference series from the National Center Research Resources (NCRR) to collaborate with institutions across the US to explore “Novel IRB Model(s) for Efficient Multisite Review”. Three large face-to-face meetings were held from June 2011 – October 2014 with more than 40 academic institutions and organizations including the Office for Human Research Protections, Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP), National Center for Advancing Translational Sciences (NCATS), Food and Drug Administration (FDA), Veterans Affairs (VA), independent IRBs, and industry (click here for more information).
- By October 2012, Vanderbilt had secured acknowledgement of compliance with 45 CFR 46.114 from OHRP (and shortly thereafter from FDA [21 CFR 56.114] and AAHRPP) and launched IRBshare to allow a single IRB review for the initial study review. After two years of piloting, IRBshare had been adopted by 45 institutions.
The initial success of IRBshare coupled with the growing use of central IRBs and regional reciprocal/ceded reliance agreements prompted the IRBshare team to once again consider expanding.
- In September 2014 Vanderbilt received an R01 from the National Heart Lung and Blood Institute (NHLBI) to empirically explore how to support and promote the use of Single IRB review. This effort was called IRBchoice, as it offered two types of reliance models: Ceded and Shared.
- By November 2015, Vanderbilt had revised the IRBshare Master Agreement to now offer both Ceded and Shared Models of reliance, plus include the IRBchoice ‘portal’ to document reliance arrangements and approvals.
- Over the course of 2016 and 2017, IRBchoice amassed nearly 80 member institutions and over 60 multi-center studies by nearly 20 different single IRBs and 44 relying institutions.
Lessons Learned and Resources Leveraged from IRBshare and IRBchoice
- Institutional Profile (IP): The IP was a major feature of the IRBchoice platform and serves as a central resource in IREx. Institutions use the IP to determine if they feel comfortable relying on, or serving as the Reviewing IRB for, other institutions. The IP became a publicly available resource on 10/1/2018. Download the IP Quick Guide.
- Study-specific Reliance Plan (SSRP): The SSRP documents how the flexible elements of oversight will be handled, for a given study. This feature was renamed for IREx and was called the Customized Action Plan (CAP) in IRBchoice. In March 2018, the SSRP was aligned with the SMART IRB Implementation Tool and Documentation Checklist.
- Study-specific Reliance Instructions: In IRBchoice, study teams responded positively to study-specific instructions the IRBchoice team generated to help participating site study teams understand how they initiate reliance at their institution and how they communicate with the single IRB, when necessary. IREx has further honed these instructions and offers a template that can be modified by any single IRB to fit with their processes and requirements. Download the template reliance instructions.